Free Printable Corrective Action And Preventive – Need one corrective action plan pattern? It discusses capa within iso 9001 and within the regulation fda 21 cfr 820 and outlines how and what data sources serve users well in. Corrective actions take steps to fix the cause of a problem after the problem has occurred, whereas preventive actions notice the problem before it occurs and takes steps to fix the cause of the problem before it happens. This results in a more compliant and streamlined capa management process.
Sample Capa Form
Free Printable Corrective Action And Preventive
Download our free, printability template and taking action today. We’ll also share a free corrective action plan template you can download and start using today. Corrective action and preventive action (capa) plan template.
A Process To Begin, Investigate, And Apply A Corrective Action Plan.
Print out the template to fill it out by hand. Preventive or corrective actions are things that are done to ensure everyone’s safety, by looking into the possible risks and taking all the necessary precautions to prevent them. Corrective action and preventive action (capa) plan template.
A Corrective Action Request Is A Formal Notification Sent To The Supplier For Rectifications To Be Done On A Nonconforming Item, Process, Or Service.
Information about resources available, constraints, stakeholders, metrics for completion, due dates, and progress updates. Download corrective and preventative action plan form template_2019.11.13. Enter the corrective action plan — a flexible system for identifying and resolving business problems.
Corrective Action “Corrective Action” Action To Eliminate The Cause Of A Detected.
Corrective action plan templates provide a structured and systematic approach to addressing problems and implementing corrective measures. Instantly format your completed corrective and preventive action reports into workflow view or register view. For additional action plan templates, check out this collection of.
Clear Establishment Of The Issues That Require This Plan.
It integrates with other quality system processes throughout the entire product life cycle. Download, print or send your reports as custom branded excel or pdf documents. Setup automated workflows for corrective action reports so that the right party is automatically notified when the report requires their digital signoff.
Clarification Of Contractor Or Team Member Responsibilities.
By using these templates, organizations can ensure that issues are addressed in a timely, consistent ,. Today’s post will provide details of these two quality management processes, with examples, templates, and an action plan. Free 7+ sample preventive action forms in ms word | pdf | excel.
This Article Covers The Important Differences Between Corrective Action And Preventive Action.
A corrective and preventive action plan (capa) will identify the source of a problem and take corrective measures to avoid recurrences. Corrective and preventive action, sometimes referred to as capa, is a quality management strategy that is made up of processes that intend to correct and prevent known issues, such as nonconformities, from occurring. Fill in the action steps necessary to reach the goal.
Corrective And Preventive Action Examples.
The capa approach is used to identify and resolve systematic defects and prevent persistent problems from happening. When a problem has been solved, it will be added to the corrective action plan. Suppliers respond to a corrective action request to report the root cause of a nonconformity and the applied corrective actions to prevent recurrence.
Corrective And Preventive Actions (Capa) Inspectional Objectives.
A corrective and preventive action plan clarifies information about standards, protocols, procedures, and ongoing compliance. When a nonconformity occurs, the laboratory. A correction can be, for example, rework or regrade iso 9000:2005(e) definition:
Use The Status Column To Easily Track And Review The Progress Of Each Problem.
Here is a simple corrective action and preventive action (capa) example: Verify that capa system procedure(s) that address the requirements of the quality system regulation have been defined and documented. A corrective action is issued to simply rectify or correct a problem or defect which has been identified or found i.e an existing non conformity a preventive action seeks to prevent a problem or non conformance issue which may not have created a defect (yet), but which has the potential to cause a problem later
In This Article, We’ll Dive Into What A Corrective Action Plan Is, Plus Why And How To Use One.

Corrective Action Plan Template Fill Online, Printable, Fillable
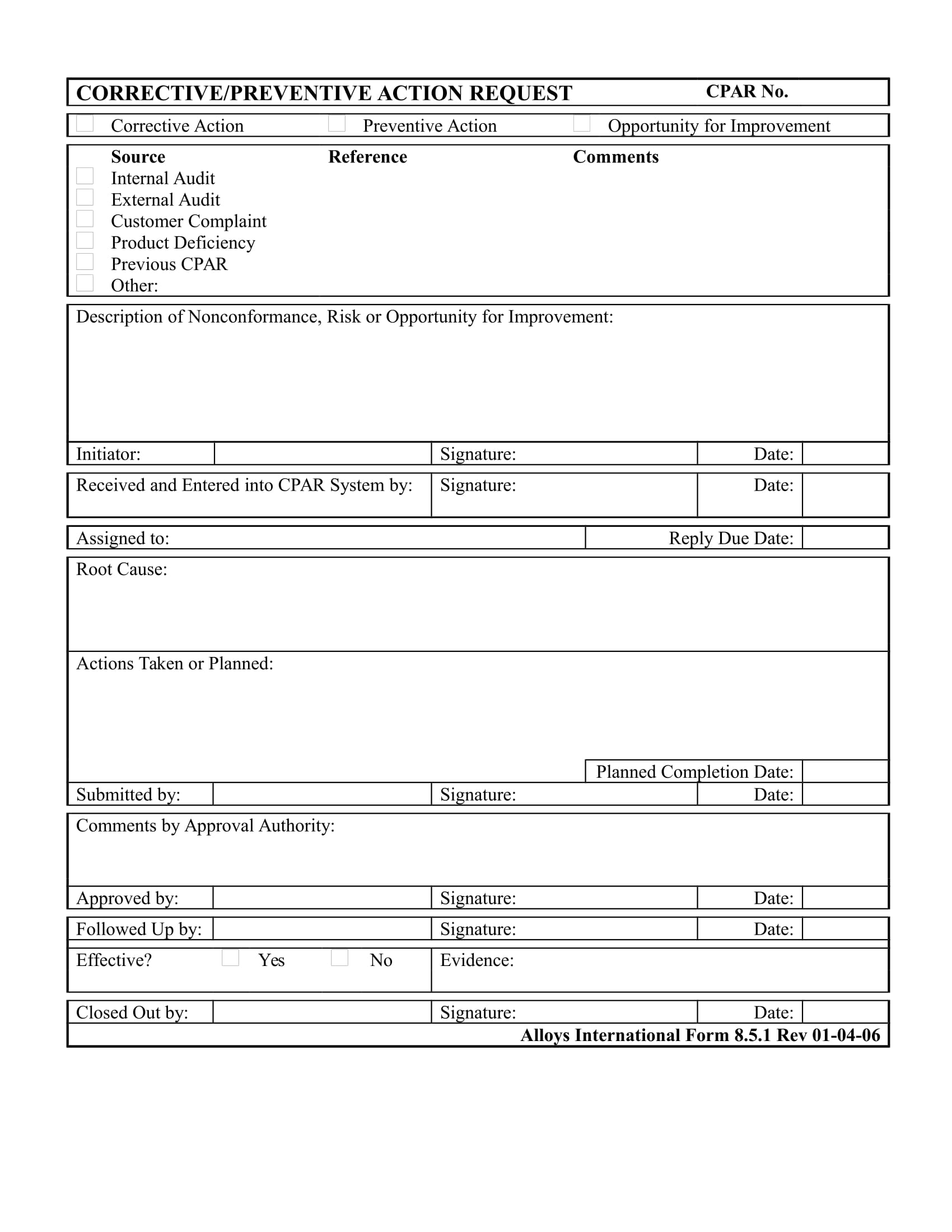
FREE 29+ Action Forms in MS Word PDF Excel

Free Corrective Action Plan Template Beautiful 8 Corrective Action

22+ Corrective Action Plan Templates Google Docs MS Word Apple

Corrective Action Plan Template 16+ Free Sample, Example, Format

Corrective and Preventive Action Format CAPA with Example

Preventive Action form Peterainsworth

FREE 10+ Sample Corrective Action Forms in PDF MS Word Excel

Corrective Action Plan Template ExcelTemplate

Free Corrective Action Plan Template Awesome 8 Corrective Action Report

Sample Capa Form

Printable Corrective Action Form Fill Online, Printable, Fillable

FREE 8+ Preventive Action Forms in PDF Ms Word Excel

FREE 8+ Preventive Action Forms in PDF Ms Word Excel

FREE 8+ Preventive Action Forms in PDF Ms Word Excel